With ultrasonic technologies as the core of Medical Business, we can quickly meet the higher level requests of medical devices.
We take up the challenges of future medical care from the field of advanced point-of-care testing.
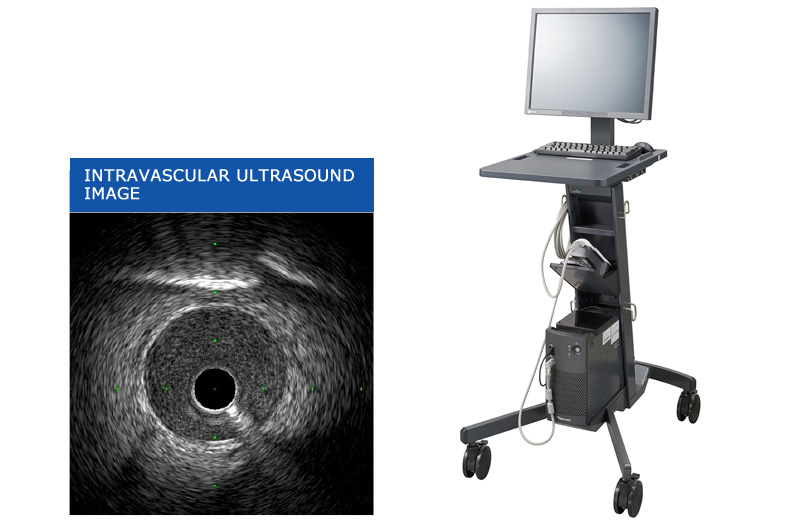
In addition to the experiences in ultrasonic diagnostic equipment, our downsizing technology of medical device can cope with the needs for the point-of-care testing in home medical care. Furthermore, our activities are with an eye to the near future, which include participation in a national-level research project such as optical ultrasonic technology.

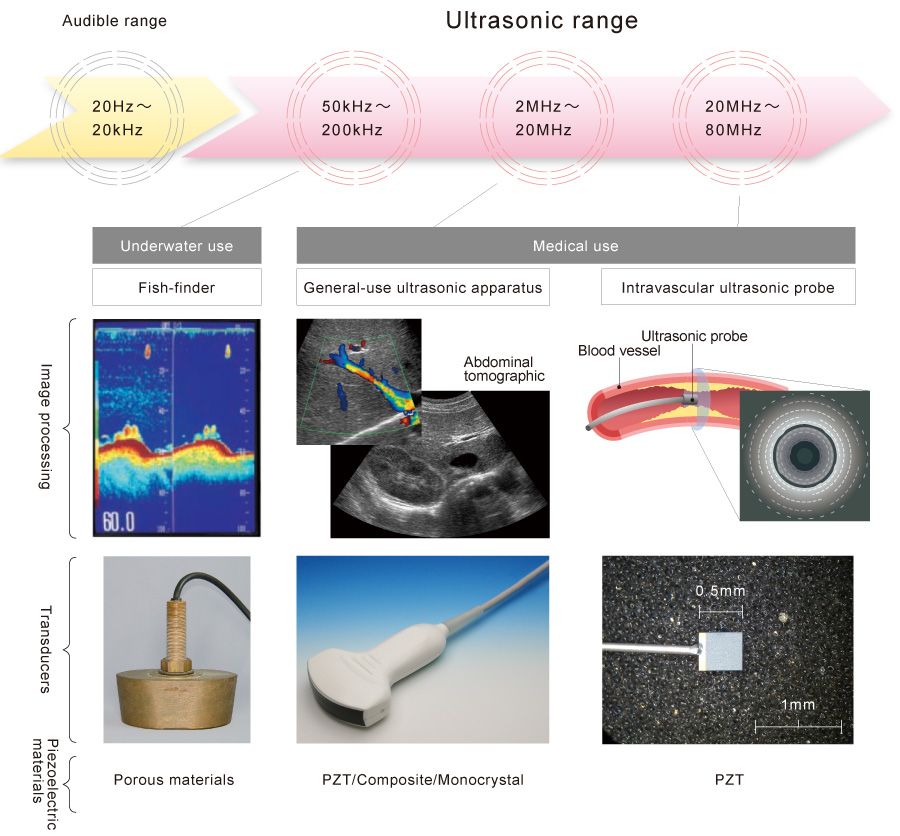
We have proficiency in manufacturing ceramics-based porous piezoelectric materials (porous materials) and piezo-composite materials – all from raw materials. We also have expertise in commercial production of ultrasonic transducers and probes used in ultrasonic diagnosis.
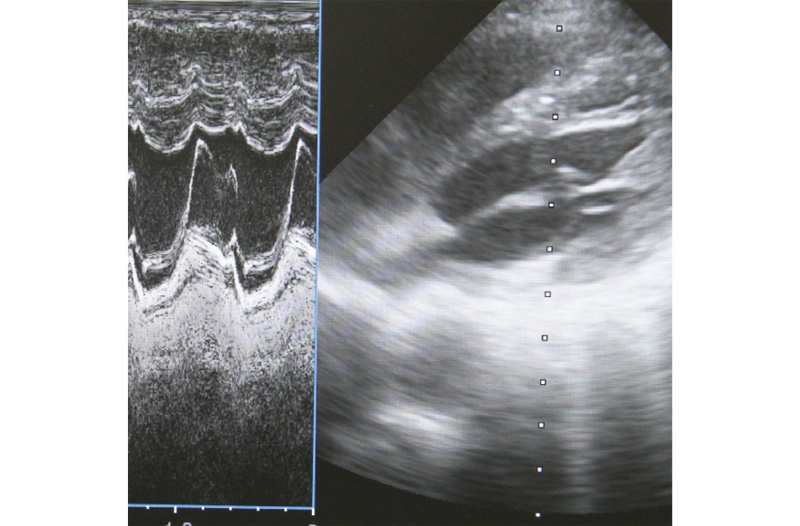
Image processing technology is used to visualize and output information obtained from ultrasonic and other types of sensors. For more accurate, precise image processing, we offer optimum algorithms by considering both hardware and software characteristics.
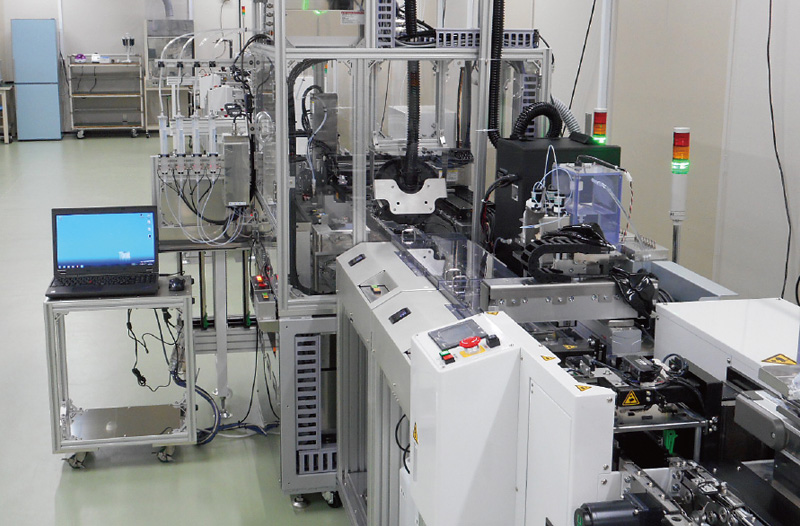
To meet the growing needs for medical-use mechatronic equipment, we design and manufacture automated blood dispensers analyzers and X-ray-equipments.
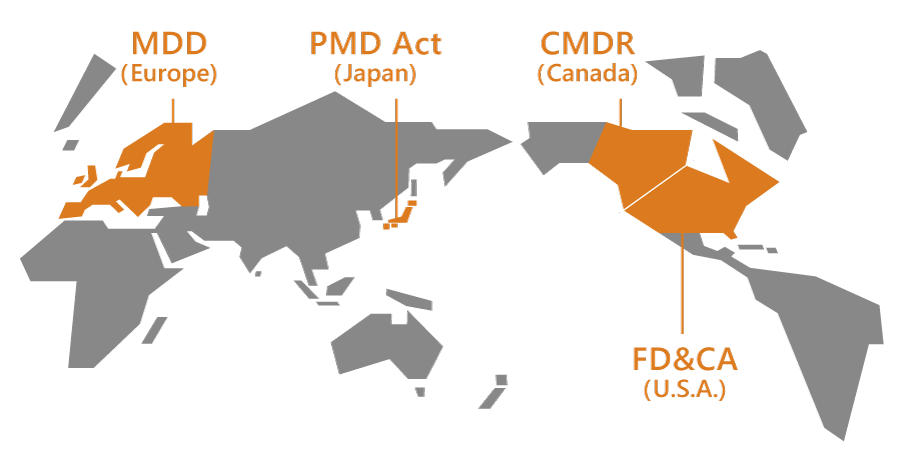
The strictest standards are set for use of medical Device in consideration of its potential negative effects on the safety of human being. However, regulations vary from country to country. Our company has the advantage of know-how that allows us to deal with such varying standards.